Oligonucleotide Synthesis
Oligonucleotide synthesis is the process of chemically synthesizing short sequences of nucleotides, typically ranging from a few bases to around 100 bases in length. These synthetic oligonucleotides can be used in various applications, including research, diagnostics, therapeutics, and biotechnology.
The synthesis of oligonucleotides involves the stepwise addition of nucleotide building blocks onto a growing chain. There are two primary methods for oligonucleotide synthesis: solid-phase synthesis and liquid-phase synthesis.
Solid-Phase Synthesis:
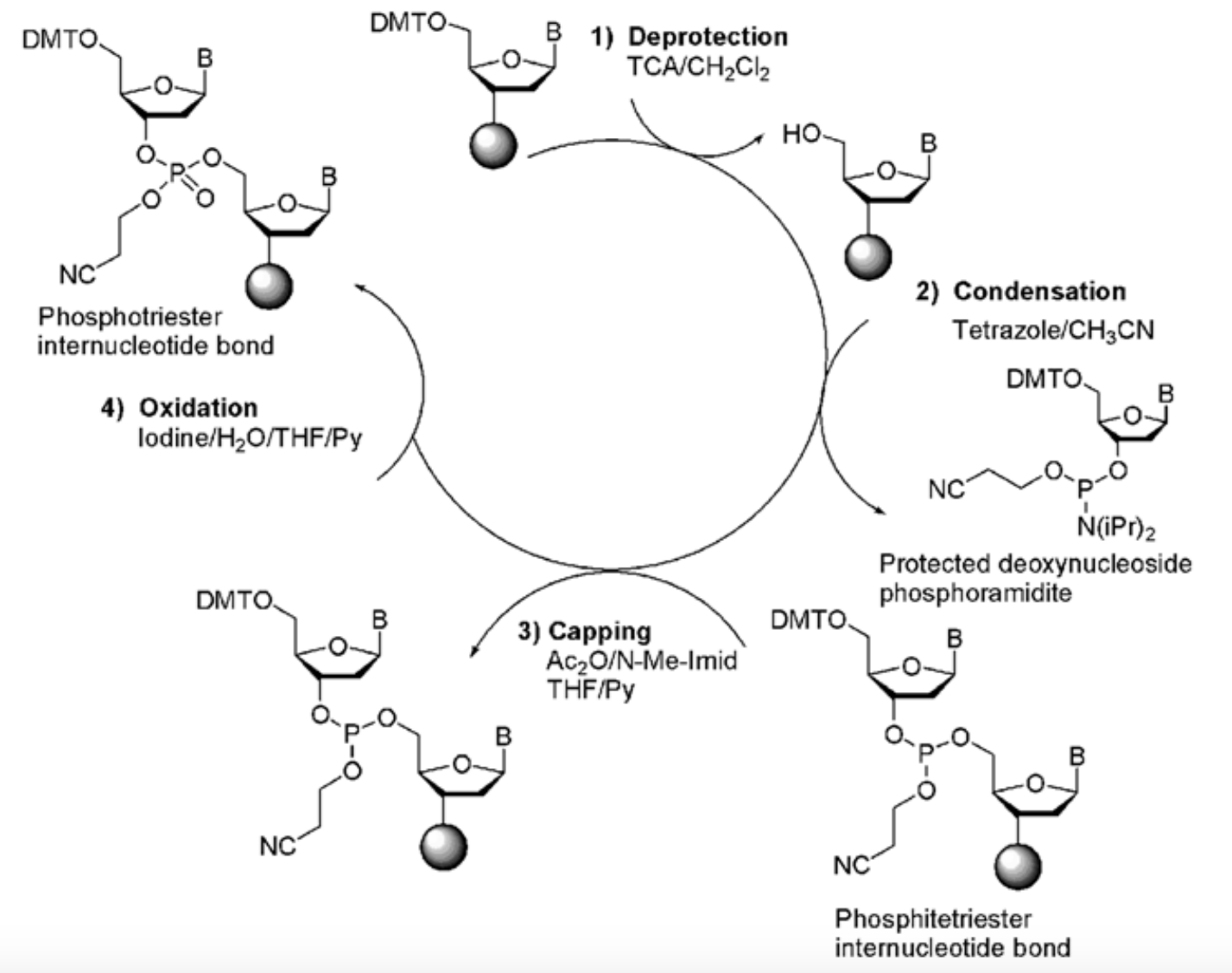
This is the most common method used for oligonucleotide synthesis. It involves anchoring the first nucleotide to a solid support, such as a polystyrene resin or controlled-pore glass bead. The nucleotide is attached to the support through a linker molecule. The synthesis proceeds in a stepwise manner, where each nucleotide is added one by one in the desired sequence. Each nucleotide addition involves protecting groups to prevent unwanted reactions. The protecting groups are removed before adding the next nucleotide. This cycle is repeated until the desired oligonucleotide sequence is obtained. After synthesis, the oligonucleotide is cleaved from the solid support and undergoes purification to remove impurities.
Liquid-Phase Synthesis:
This method involves the synthesis of oligonucleotides in a solution phase rather than on a solid support. It is typically used for shorter oligonucleotides or for specialized applications. In liquid-phase synthesis, nucleotides are coupled together in solution using specific reagents and reaction conditions. The reaction mixtures are carefully controlled to prevent unwanted side reactions and to ensure high yields and purity. After synthesis, the oligonucleotide is purified through techniques such as ethanol precipitation or chromatography.
In both methods, the nucleotide is protected at various functional groups to prevent unwanted reactions during the synthesis. The most commonly used protecting group for the 5′-hydroxyl group of the nucleotide is a dimethoxytrityl (DMT) group. The 3′-hydroxyl group is usually protected with a base-labile group, such as a β-cyanoethyl group.
After the first nucleotide is attached, a series of steps known as coupling, capping, and oxidation are performed iteratively to add subsequent nucleotides. During the coupling step, a nucleotide phosphoramidite, which is protected at its reactive groups, is reacted with the growing chain, resulting in the formation of a phosphodiester bond. Any unreacted sites on the solid support are capped to prevent unintended reactions, and oxidation ensures the stability of the phosphodiester linkage.
After the desired oligonucleotide sequence is assembled, it undergoes a series of deprotection steps to remove the protecting groups from the nucleotides. This allows the oligonucleotide to be cleaved from the solid support and purified using techniques such as high-performance liquid chromatography (HPLC) or cartridge-based purification systems.
Once purified, the synthesized oligonucleotide can be used in a variety of applications, including PCR, DNA sequencing, gene expression analysis, gene synthesis, RNA interference (RNAi), and gene editing technologies like CRISPR-Cas9.
Oligonucleotide synthesis is a crucial technique in molecular biology and various applications, including genetic research, diagnostics, therapeutic development, and biotechnology. It enables the generation of custom-designed DNA or RNA sequences for specific experimental or practical purposes.
